Cholinesterase, AcetylI.U.B.: 3.1.1.7 Enzymatic Reaction (image will open in a new window) Acetylcholinesterase (AChE) catalyzes the hydrolysis of acylcholinesters with a relative specificity for acetylcholine: The enzyme is bound to cellular membranes of excitable tissue (synaptic junction, endoplasmic reticulum, etc.) and is believed to be associated with nerve impulse conduction (Politoff et al; 1975, Friedenberg and Seligman 1972, Nachmansohn 1970). AChE is also found in red blood cells. A second cholinesterase found in blood serum hydrolyzes butyrylcholine 4 times faster than acetylcholine. They are two distinct enzymes. See monograph on butyrylcholinesterase (E.C.3.1.1.8). See also, Chatonnet and Lockridge (1989). Enzymatic and immunochemical properties of AChE from electric eel have been reported by Gurari et al. (1974) and Leuzinger (1971). See also Sadar and Laidler (1975), Jain et al. (1973), Robaire and Kato (1973), Dudai et al. (1972), Rosenberry and Bernhard (1972), and Froede and Wilson (1971). Other reported sources of AChE are: mouse brain (Adamson et al. 1975); houseflies (Devonshire 1975); venom (Kumar and Elliott 1973); pig brain (McIntosh and Plummer 1973); human erythrocytes (Sihotang 1974; Chajilani, et al. 1989; Paniker et al. 1973); rat liver (Wheeler et al. 1972); and mollusc (Bevelaqua et al. 1975). See also Krupka and Hellenbrand (1974) and Ngo and Laidler (1975a and b). Goodson et al. (1973) report on immobilized AChE. Characteristics of AChE from Electric Eel:Molecular weight: 260,000 (Leuzinger et al. 1969). Composition: Levinson and Ellory (1974) indicate that the enzyme in its natural state is a monomer of approximately 75,000; in solution, aggregates are present. Rosenberry et al. (1974) report subunits to have a molecular weight of 70,000 and one active site. See also Chen et al. (1974) and Berman (1973). Dudai et al. (1973) have also reported on molecular structure. There is evidence that eel AChE is a glycoprotein (Bon and Rieger 1975; Powell et al. 1973). Mooser and Sigman (1974 and 1972) report on a non-catalytic ligand binding-site remote from the active site. See also: Cocolas et al. (1974); Fuchs et al. (1974); Hochachka (1974); Marquis and Webb (1974); Roskowski (1974); Kato et al. (1972); Mooser et al. (1972); Rieger et al. (1972); Rosenberry (1975) and Rosenberry et al. (1972). Optimum pH: 7.0. Extinction coefficient:
Isoionic point: pH 5.35. Specificity: At substrate concentrations of 1 X 10-3M and constant enzyme level (approximately optimal for acetylcholine), relative rates of hydrolysis for several esters are: acetylcholine, 100; propionylcholine, 96; butyrylcholine, negligible; and triacetin, 11 (Nachmansohn 1959). See also Moore and Hess (1975). Inhibitors: The classical inhibitors of AChE are organophosphate compounds (Bartels and Nachmansohn 1969 and Ashani et al. 1972) Most carbamates inhibit it (Hetnarski and O'Brian 1975). Light-sensitive inhibitors have been studied by Deal et al. (1969). See also: Maheshwari et al. (1975); Reiner et al. (1975); Millner et al. (1974); Stanley et al. (1974); deJong and vanDijk (1972); Allen and Abeles (1989). Moss et al. (1974) report on its reaction with puromycin. Activator: 0.02 M Mg2+ is stimulatory in purified preparations. Stability: The enzyme is stable as a lyophilized powder for months at -20deg.C and in solution at pH 7.0 for several days at 4deg.C. Reports on methods of assay include the following: Schnitzerling and Nolan (1975); Stoops and Bender (1975); Augustinsson and Eriksson (1974); Lewis and Eldefrawi (1974); Smith (1974); Ellin et al. (1972); Vete et al. (1972); and vanHooidonk et al. (1972). See also comments by Ellin (1972). |

 Place Order
Place Order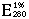 = 16.1 (Leuzinger et al. 1968).
= 16.1 (Leuzinger et al. 1968).